Synthetic cell demonstrations
This page provides an overview of synthetic cell demonstrations reported in the scientific literature. The information was generated using a prompt requesting examples of synthetic cell demonstrations from specific research groups, processed by Claude Sonnet 4 on August 29, 2025. The examples focus on bottom-up approaches to creating artificial cellular systems with behaviors that can be incorporated into more complex synthetic cell-based systems.
Vesicle-based demonstrations
This section describes selected examples of synthetic cell-based systems where the compartment is a lipid bilayer vesicle.
Engineering Genetic Circuit Interactions Within and Between Synthetic Minimal Cells (2017)
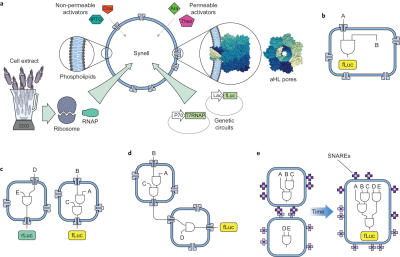
Adamala and Boyden demonstrated the first robust example of genetic circuit-based communication between populations of synthetic cells. Their system used liposome-encapsulated genetic circuits that they termed "synells" (synthetic minimal cells). The most sophisticated demonstration involved two distinct populations: sensor synells containing IPTG and genetic circuits to produce α-hemolysin, and reporter synells containing circuits that responded to released signaling molecules (doxycycline and IPTG) by expressing firefly luciferase. The α-hemolysin created pores in membranes, allowing controlled release of signaling molecules and establishing cascaded communication between the two cell populations without crosstalk.
Adamala, K. P., Martin-Alarcon, D. A., Guthrie-Honea, K. R., & Boyden, E. S. (2017). Engineering genetic circuit interactions within and between synthetic minimal cells. Nature Chemistry, 9(5), 431-439.
Cell-Sized Mechanosensitive and Biosensing Compartment Programmed with DNA (2017)
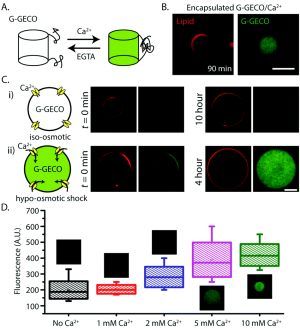
Liu's group at the University of Michigan, in collaboration with Vincent Noireaux at the University of Minnesota, demonstrated synthetic cells capable of coupling mechanical input to biosensing through genetically programmed components. The system used liposomes containing cell-free transcription-translation (TX-TL) reactions that expressed two key proteins: the E. coli mechanosensitive channel of large conductance (MscL) and the calcium biosensor G-GECO. They showed that osmotic pressure changes could activate MscL channels in the synthetic cell membrane, allowing calcium influx that was detected by the co-expressed G-GECO protein through a 23-26 fold increase in fluorescence.
Majumder, S., Garamella, J., Wang, Y. L., DeNies, M., Noireaux, V., & Liu, A. P. (2017). Cell-sized mechanosensitive and biosensing compartment programmed with DNA. Chemical Communications, 53(53), 7349-7352.
Controlling Secretion in Artificial Cells with a Membrane AND Gate (2019)
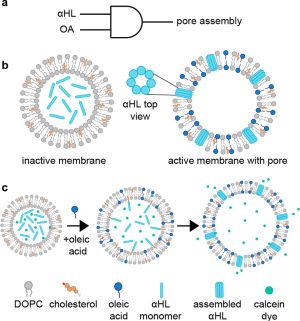
Kamat's group at Northwestern developed artificial cells capable of controlled secretion using a membrane-based AND gate that implements Boolean logic through membrane composition changes. The system used giant unilamellar vesicles containing α-hemolysin protein and required both oleic acid (fatty acid) AND the pore-forming protein to achieve cargo release. The key innovation was controlling when α-hemolysin could functionally assemble into membrane pores based on membrane lipid composition. Initially, vesicles with low oleic acid content prevented α-hemolysin from assembling into functional pores, keeping the membrane impermeable. However, when oleic acid micelles were added externally, they incorporated into the vesicle membrane, changing its composition to enable α-hemolysin assembly into functional heptameric channels. This membrane transformation triggered the release of encapsulated cargo such as calcein. The system demonstrated that membrane-based Boolean logic could complement genetic circuits and provided a new method for temporal control of vesicle permeability through membrane protein-lipid interactions.
Hilburger, C. E., Jacobs, M. L., Lewis, K. R., Peruzzi, J. A., & Kamat, N. P. (2019). Controlling secretion in artificial cells with a membrane AND gate. ACS Synthetic Biology, 8(6), 1224-1230.
TXTL-Based Synthetic Cell Systems (2021)
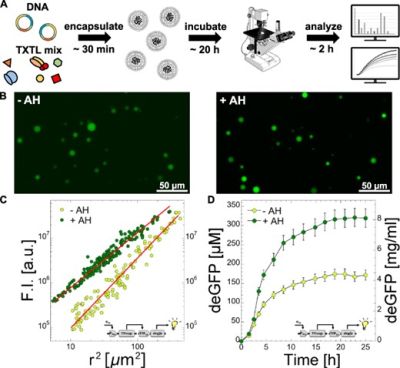
Noireaux's group developed the all-E. coli TXTL toolbox 3.0 for creating synthetic cell prototypes using cell-free transcription-translation systems. The most sophisticated demonstration involved semi-continuous synthetic cells where liposomes loaded with TXTL reactions could produce enhanced green fluorescent protein (eGFP) at concentrations exceeding 8 mg/ml. This was achieved by allowing chemical building blocks to diffuse through membrane channels, creating a feeding mechanism that sustained protein synthesis over extended periods. The system also demonstrated the synthesis of complex biological entities, including the complete bacteriophage T7 (40 kb genome, ~60 genes) at concentrations of 10^13 PFU/ml.
Garenne, D., Thompson, S., Brisson, A., Khakimzhan, A., & Noireaux, V. (2021). The all-E. coli TXTL toolbox 3.0: new capabilities of a cell-free synthetic biology platform. Synthetic Biology, 6(1), ysab017.
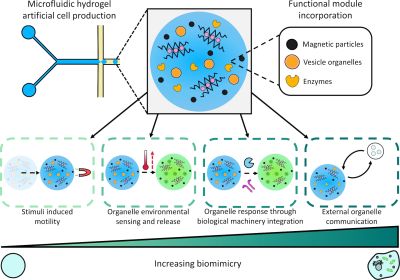
Biomimetic Behaviours in Hydrogel Artificial Cells through Embedded Organelles (2023)
Elani's group at Imperial College developed a microfluidic strategy to create biocompatible cell-sized hydrogel-based artificial cells with embedded functional subcompartments acting as engineered synthetic organelles. They demonstrated artificial cells capable of multiple biomimetic behaviors through modular, interchangeable subcompartments. The system included magnetic particles as "motility organelles" that enabled stimulus-induced movement when exposed to magnetic fields, and lipid vesicle organelles containing encapsulated cargo that could be released in response to specific enzymatic biomarkers. For example, vesicles containing β-galactosidase substrate were embedded within the hydrogel matrix, and when the enzyme was present in the environment, it triggered controlled release of the vesicle contents. The artificial cells also demonstrated enzymatic communication with surrounding bioinspired compartments through cascaded biochemical reactions. This work represents a demonstration of hydrogel-based artificial cells with multiple types of synthetic organelles that could replicate complex cellular behaviors including motility, sensing, content release, and intercellular communication within a single synthetic cell chassis.
Allen, M. E., Hindley, J. W., O'Toole, N., Cooke, H. S., Contini, C., Law, R. V., ... & Elani, Y. (2023). Biomimetic behaviours in hydrogel artificial cells through embedded organelles. Proceedings of the National Academy of Sciences, 120(35), e2307772120.
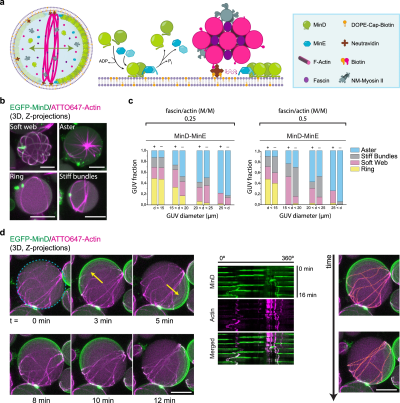
Self-Organized Spatial Targeting of Contractile Actomyosin Rings for Synthetic Cell Division (2024)
Schwille's group at the Max Planck Institute were able to implement synthetic cell division by successfully combining eukaryotic actomyosin contractile machinery with the bacterial MinDE protein positioning system. The most sophisticated demonstration showed giant unilamellar vesicles containing actomyosin rings that were spatiotemporally positioned at the vesicle equator by MinDE pole-to-pole oscillations through a diffusiophoretic transport mechanism. The MinDE system created active transport of the membrane-bound actomyosin structures via frictional forces, accumulating them at mid-cell in an orientation perpendicular to the oscillation axis. The positioned contractile rings then generated sustained furrow-like membrane invaginations, breaking the spherical symmetry and creating two-lobed vesicles while maintaining the spatial organization. This work represented the first successful integration of spatial positioning machinery with contractile elements to achieve controlled membrane constriction at defined locations, addressing one of the key challenges in synthetic cell division.
Reverte-López, M., Kanwa, N., Qutbuddin, Y., et al. (2024). Self-organized spatial targeting of contractile actomyosin rings for synthetic cell division. Nature Communications, 15, 10415.
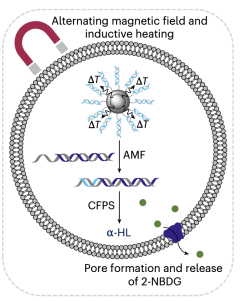
Magnetic Activation of Spherical Nucleic Acids for Remote Control of Synthetic Cells (2025)
Booth's group at the University of Oxford and University College London developed synthetic cells controlled by clinically tolerable magnetic fields using spherical nucleic acids with magnetic nanoparticle cores. The system used DNA promoter sequences attached to silica-encapsulated iron oxide nanoparticles that could be activated by alternating magnetic fields at 100 kHz—the only clinically approved frequency for magnetic hyperthermia. When exposed to the magnetic field, localized heating from the nanoparticles released T7 promoter sequences that activated cell-free protein synthesis within giant unilamellar vesicles. The most sophisticated demonstrations showed magnetically controlled expression of mNeonGreen fluorescent protein and α-hemolysin pore-forming protein, which enabled on-demand cargo release from synthetic cells. The system maintained tight control with minimal background activity in the absence of magnetic fields through a novel purification method that removed electrostatically bound DNA. Critically, the technology operated through opaque blocking materials that are impenetrable to current activation methods like light or small molecules, demonstrating the potential of deeply tissue-penetrating magnetic fields for controlling synthetic cells as drug delivery devices.
Parkes, E., Al Samad, A., Mazzotti, G., Newell, C., Ng, B., Radford, A., & Booth, M. J. (2025). Magnetic activation of spherical nucleic acids enables the remote control of synthetic cells. Nature Chemistry (published online).
Other compartmentalization techniques
This section describes selected examples of systems where the compartment is something other than a lipid bilayer-based vesicle.
Light-Activated Communication in Synthetic Tissues (2016)
Booth and colleagues developed one of the earliest demonstrations of communication between droplet-based synthetic cells using light-activated control systems. The system involved 3D-printing droplets containing PURE cell-free transcription-translation (TX-TL) systems that could produce α-hemolysin pore proteins upon light activation. When these pore proteins were incorporated into specific bilayer interfaces, they mediated rapid, directional electrical communication between subsets of artificial cells, mimicking neural transmission in living tissue. The light activation provided precise spatial and temporal control over which cells could communicate, creating the first demonstration of tissue-like organization in synthetic cell systems.
Booth, M. J., Schild, V. R., Graham, A. D., Olof, S. N., & Bayley, H. (2016). Light-activated communication in synthetic tissues. Science Advances, 2(4), e1600056.
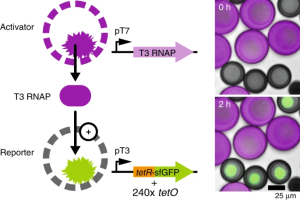
Communication and Quorum Sensing in Artificial Cells (2018)
Devaraj’s group at UC San Diego developed artificial cells capable of chemical communication using quorum-sensing genetic circuits encapsulated inside a porous polymer membrane containing an artificial hydrogel compartment. "Activator" synthetic cells produced T3 RNAP, which diffused through the polymer membrane into "detector" synthetic cells that expressed GFP. The system demonstrated that non-living vesicle-based mimics could exchange information through diffusible signaling molecules, allowing one population of artificial cells to regulate gene expression in another. This work provided a demonstrations of intercellular communication between synthetic cells and highlighted the potential of quorum sensing as a design principle for coordinating behavior in cell-free systems.
Niederholtmeyer, H., Chaggan, C., & Devaraj, N. K. (2018). Communication and quorum sensing in non-living mimics of eukaryotic cells. Nature Communications, 9, 5027.
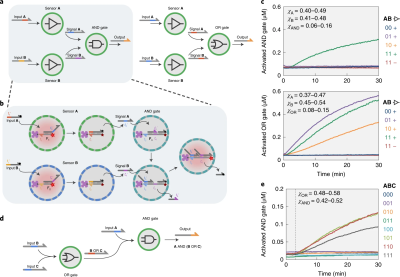
DNA-Based Communication in Populations of Synthetic Protocells (2019)
Joesaar, Mann, de Greef and colleagues developed a highly sophisticated platform called "biomolecular implementation of protocellular communication" (BIO-PC) using proteinosomes as artificial cell chassis. The system leveraged enzyme-free DNA strand-displacement circuits encapsulated within semipermeable protein-polymer microcapsules. The most complex demonstration showed bidirectional communication and distributed computational operations, where protocells could sense DNA-based input messages, process them through programmable logic circuits, and secrete output DNA strands that activated neighboring protocells. The encapsulation protected the DNA circuits from nuclease degradation, allowing operation in concentrated serum conditions.
Joesaar, A., Yang, S., Bögels, B., van der Linden, A., Pieters, P., Kumar, B. V. V. S. P., ... & de Greef, T. F. A. (2019). DNA-based communication in populations of synthetic protocells. Nature Nanotechnology, 14(4), 369-378.